National Administration of Drugs, Foods & Medical Devices of Argentina (ANMAT)
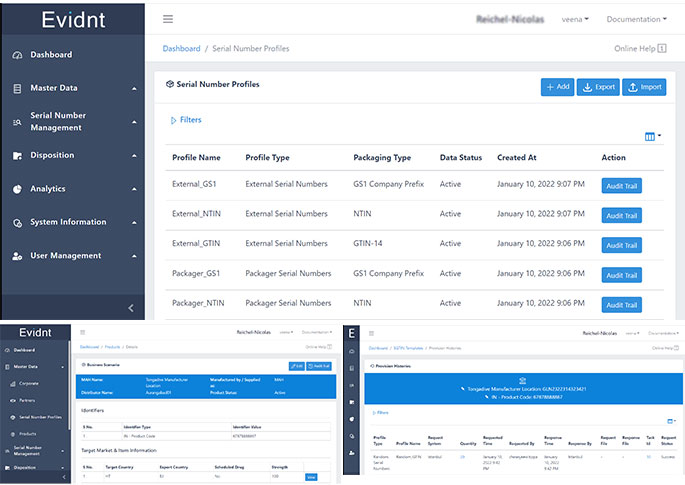
Evidnt
Evidnt by Tongadive is a cutting-edge supply chain traceability software designed to provide end-to-end visibility and secure tracking of products. Leveraging technologies like GS1 Serialization, Blockchain Distributed Ledger Technology (DLT), and Neural Networks, it ensures complete transparency, traceability, and accountability throughout the product lifecycle. This software has versatile applications across industries including pharmaceuticals, agriculture, food processing, and sustainable fashion. Available both as a cloud-based SaaS or on-premises system, Evidnt offers flexible deployment to suit a wide range of business needs, enhancing product integrity and consumer trust.
Request a Demo
Download a brochure
Where does Evidnt Add Value
Regulatory Compliance on supply chain visibility in regulated industries like Pharmaceutical, Medical Devices, Agriculture and Food Processing

L4, L5 and Next Generation supply chain tracebility & visibility Software enabling regulatory compliance in industries like Pharmaceutical, Medical Devices, Agriculture and Food Processing

Cost efficiency through end to end supply chain tracebility & visibility Software supporting ‘Just in Time’ production through better demand forecasting
Waste reduction promoting supply chain traceability software sustainability through visibility of demand and consumption at transaction level

Circularity and Re-commerce through trustworthy product origin information

Ethical and sustainable sourcing promotion through Provenance tracking
Unique Features of Evidnt

Unique Algorithm
Innovative algorithm that generates GS1 Serialised data and tracks it on Blockchain DLT Platform with an ability to run intelligent analytics and connection to Neural Networks based predictive demand forecasting information.

IT Systems
No fuss Deployment with plug & play approach supported by Tongadive expert team without having to make extensive changes in existing enterprise systems

User Experience
Simple and Intuitive end user experience making track and trace operations fun and less prone to errors

Automation
Compliance Audit on a Click enabled through automations reducing time and complexity of adherence to audit requirements and exposure to penalties

Flexibility/ Adaptability
Flexibility to adapt and tailor to supply chain stakeholder requirements through Cloud based microservices architecture
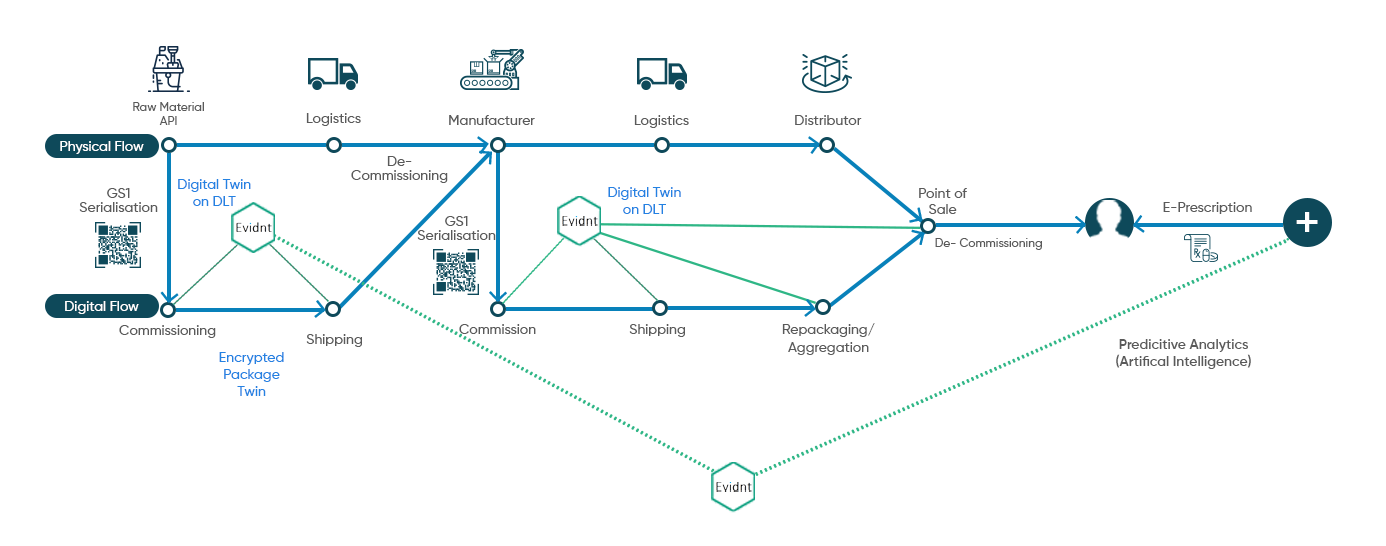
Digital Twin based Journey Tracking
Evidnt helps create a digital twin of product package and tracks
its journey from the source to the
end consumer and beyond.
Consulting and IT Services

GS1 Serialisation based Compliance
With extensive experience in digital technologies, compliance requirements across sectors, and deep industry experience, Tongadive offers tailored services to help organizations meet track and trace obligations. By leveraging its supply chain traceability software, businesses can ensure compliance while expanding operations to new countries or meeting evolving regulations in existing markets. Tongadive specializes in developing and implementing serialization strategies, empowering companies to stay ahead of global standards and regulatory requirements with ease.
- US Drug Supply Chain Security Act (DSCSA)
- European Falsified Medicine Directive (FMD)
- UK Medicines and Healthcare Regulatory Agency (MHRA)
- India iVEDA portal (Integrated Validation of Exports of Drugs from India and its Authentication)
- Other Existing and Emerging Compliance Markets
Track and Trace based Packaging Solutions
Tongadive experts can help develop and operationalise track and trace strategy aimed at achieving business varied objectives.
- Visibility of products across supply chain
- Counterfeit avoidance strategies
- Cost efficiency in supply chain operations
- Demand forecasting for optimising production
- Supply chain software deployment support
- L1-L5 packaging and track and trace strategies
- Track and trace managed services
- Sustainability and re-commerce focused track and trace strategies
- Circularity protocols
Consultancy Services – Medical Devices
Our Consultancy services for medical devices, provide valuable support and expertise in navigating the regulatory landscape, market entry strategies, quality management systems, Manufacturing infrastructure, Product Development, supply chain traceTechnology transfer, Clinical Trials of Medical Devices, Drugs / pharma, and DPR for overall business development. Here are some key areas where consultancy services can assist:

A. Regulatory Consultancy
- Regulatory Compliance: Guidance through the complex regulatory requirements for medical devices in India, including classification, product registration, Test license, manufacturing license import licensing, and obtaining necessary approvals from regulatory bodies like CDSCO
- Quality Management Systems (QMS): Help establish and implement QMS to comply with Indian regulations and international standards such as ISO 13485. We can assist with QMS documentation and support in risk management, internal audits etc
- Product Development and Design Control: Providing expertise in product development, design control processes, and usability files. Help in developing a product that meets user needs, safety requirements, and regulatory standards.
- Clinical / Performance Evaluation and Studies: Planning and conducting clinical studies or evaluations required for medical device approvals in India. Help to design study protocols, identify suitable study centers, and guidance through the ethical and regulatory requirements.
- Post-Market Surveillance and Vigilance: Providing necessary support for the establishment of post-market surveillance systems, adverse event reporting, complaint handling processes, and vigilance activities to ensure ongoing compliance and product safety.
- Product Registration and Market Launching- All medical devices should adhere to country-specific regulatory compliances, provide guidance on regulatory requirements, assist with preparing regulatory submissions, help with pre-market approvals (such as FDA 510(k) or CE marking), and support compliance with international standards like ISO 13485.
- Global certifications- Dossier preparation and review of documents for attaining certifications for WHO, CE, USFDA, MHRA etc.
- Research and Development certification- Provide all necessary support in filing for DSIR certification
- Packaging and labelling- Packaging of medical devices to involve key aspects like product protection, sterility, material selection, device integrity and labelling. Packaging may incorporate labels or printing that provides important information about the device, such as its name, description, instructions for use, warnings, and regulatory symbols in compliance with ISO 15223, ISO 11607, and ISO 20417. UDI is another important factor of consideration
- Risk Management-Risk management of medical devices is a crucial aspect of ensuring patient safety and regulatory compliance. It involves identifying, assessing, and mitigating risks associated with the design, development, manufacturing, and use of medical devices. We assist in documentation of risk management in compliance to regulatory standard ISO 14971.
- Post Market regulatory activities- Establish procedures for evaluating and implementing corrective and preventive actions (CAPA) based on post-market surveillance data. These actions may include device recalls, design modifications, or additional safety measures to address identified risks and updating of required documents.
- Audit Preparation and Post audit support: Preparation of your company for the audit by conducting internal audits or pre-audit assessments. Review the quality management system, processes, and documentation to identify any gaps or potential non-compliance issues.
B. Manufacturing and Infrastructure
-
Facility Design and Layout- Designing the layout of manufacturing facilities, taking into consideration factors such as workflow efficiency, cleanroom requirements, equipment placement, and safety considerations. Provide recommendations for facility infrastructure, utilities, and environmental controls to meet the specific needs of medical device manufacturing.
- Regulatory Compliance- Manufacturing processes and infrastructure adhere to relevant standards, such as ISO 13485 (Quality Management Systems for Medical Devices) and FDA regulations.
- Manufacturing Strategy- Determining the optimal manufacturing processes, technologies, and equipment required for efficient production. Identifying potential manufacturing partners or optimizing in-house manufacturing capabilities.
- Process Validation- Validation of manufacturing processes, develop validation protocols, conduct process performance qualifications (PPQ), and establish appropriate monitoring and control mechanisms.
- Supply Chain Management- Optimize the supply chain for medical device manufacturing, including supplier selection, materials sourcing, inventory management, and logistics, timely delivery of components and raw materials while maintaining quality and cost efficiency.
- Continuous Improvement- Implementing continuous improvement methodologies, such as Lean Manufacturing or Six Sigma, to enhance productivity, reduce waste, and increase overall operational efficiency, process optimization, cost reduction, and quality enhancement.
C. Technology Transfer
- Technology Assessment- Evaluate the existing medical device technology to determine its suitability for transfer. This includes assessing the technology’s intellectual property, market potential, regulatory compliance, and manufacturing feasibility.
- Due Diligence- due diligence to assess the risks and benefits associated with the technology transfer. This involves analyzing legal, regulatory, and intellectual property aspects, as well as evaluating the financial viability of the transfer.
- Manufacturing Process Optimization- Optimize the manufacturing process of the medical device to enhance efficiency, reduce costs, and improve quality control. reviewing and refining production processes, equipment selection, and implementing quality management systems.
- Knowledge Transfer and Training- Facilitate transfer of knowledge and expertise from the technology provider to the recipient organization. develop training programs and materials to ensure the successful adoption and utilization of the transferred technology.
- Project Management- project management expertise to ensure a smooth and efficient technology transfer process. They develop detailed project plans, coordinate activities between stakeholders, and monitor progress to meet timelines and milestones.
- Market Entry Strategy- Market entry strategy for the recipient organization, considering factors such as target markets, competitive landscape, pricing, distribution channels, and marketing plans
- Risk Management- Address potential risks associated with technology transfer, including intellectual property protection, regulatory compliance, supply chain vulnerabilities, and quality control issues.
D. Detailed Project Report (DPR)
- To provide a comprehensive overview of the development process and feasibility of a medical device. The report outlines the objectives, market analysis, supply chain tracebility software ,technical specifications, manufacturing plan, regulatory considerations, financial projections, Project Timelines, and other important insights of project
Serialization. Traceability. Compliance
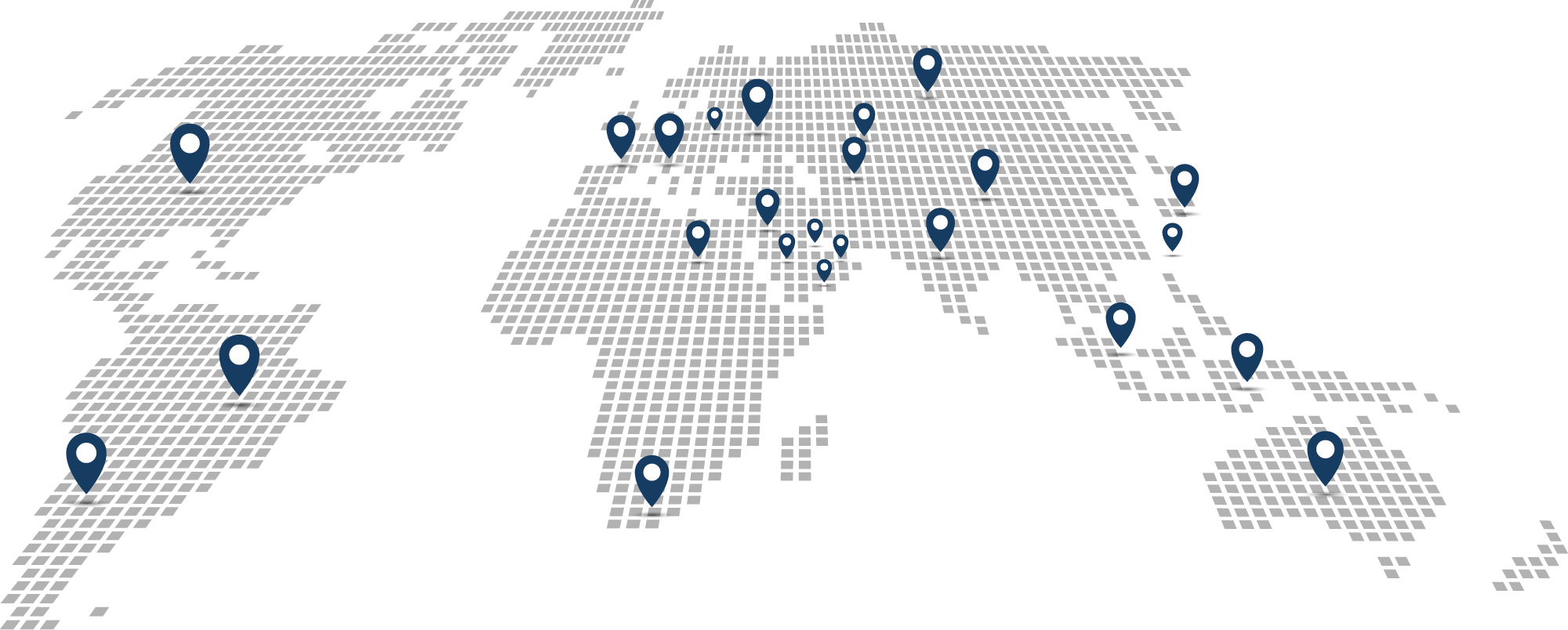
 Argentina
ArgentinaNational Administration of Drugs, Foods & Medical Devices of Argentina (ANMAT)
- Required
- GS1-128, DataMatrix, RFID
- https://www.argentina.gob.ar/anmat
We are ready to serve !
 Australia
AustraliaDepartment of Health, Therapeutic Goods Administration
- Must comply by Jan. 1, 2023
- DataMatrix
- https://www.tga.gov.au
We are ready to serve !
 Bahrain
BahrainNational Health Regulatory Authority (NHRA)
- Required
- DataMatrix
- https://www.nhra.bh/
We are ready to serve !
 Brazil
BrazilBrazilian Health Regulatory Agency (ANVISA)
- Must comply by April 1, 2022
- DataMatrix
- https://www.gov.br/
We are ready to serve !
 China
ChinaNational Medical Products Agency (NMPA)
- No deadline announced
- Linear barcode, DataMatrix, RFID, HRI
- http://english.nmpa.gov.cn/
Coming Soon !
 Egypt
EgyptCentral Administration for Pharmaceutical Affairs (CAPA)
- No deadline announced
- DataMatrix
- http://www.eda.mohp.gov.eg/
We are ready to serve !
 India
IndiaMinistry of Commerce & Industry
- Required now
- DataMatrix
- https://commerce.gov.in/
We are ready to serve !
 Indonesia
IndonesiaNational Agency of Drug and Food Control
- Must comply by Jan. 1, 2023
- QR Code or DataMatrix
- GTIN or AI (distribution permit number) Serial number Expiry date…
GTIN or AI (distribution permit number)
Serial number
Expiry date
Batch/LotRequirements at tertiary, secondary & primary (including SN) levels
Requirement on primary packaging only if there is not an anti-tampering device on the packaging level above
- https://www.pom.go.id/new/home/en
We are ready to serve !
 Japan
JapanMinistry of Health, Labor, and Welfare
- No deadline announced
- N/A
- https://www.mhlw.go.jp/english/
We are ready to serve !
 Kazakhstan
KazakhstanMinistry of Healthcare
- No deadline announced
- DataMatrix
- https://www.gov.kz/
We are ready to serve !
 Lebanon
LebanonMinistry of Health MedTrack
- Does not seem required
- DataMatrix
- https://www.moph.gov.lb/en/
We are ready to serve !
 Malaysia
MalaysiaMinistry of Health of Malaysia
- Must comply by Jan. 1, 2023
- DataMatrix
- https://www.moh.gov.my/
We are ready to serve !
 Qatar
QatarMinistry of Public Health
- Does not seem required
- DataMatrix (secondary); DataMatrix or GS1 128 (tertiary)
- https://www.moph.gov.qa/
We are ready to serve !
 Saudi Arabia
Saudi ArabiaSaudi Food & Drug Authority (SFDA)
- Active
- DataMatrix
- https://www.sfda.gov.sa/en
We are ready to serve !
 South Africa
South AfricaNational Department of Health
- Active
- DataMatrix
- https://www.health.gov.za/
We are ready to serve !
 Switzerland
SwitzerlandSwiss Medicines Verification Organization (SMVO)
- Active
- DataMatrix
- https://smvo.ch/en/
We are ready to serve !
 United Arab Emirates
United Arab EmiratesMinistry of Health and Prevention
- Active
- DataMatrix
- https://mohap.gov.ae/en/
We are ready to serve !
 Ukraine
UkraineMinistry of Eduction and Science
- No deadline announced
- DataMatrix
- https://mon.gov.ua/eng
We are ready to serve !
 United States
United StatesFood and Drug Administration
- Must comply by Nov. 27, 2023
- DataMatrix
- https://www.fda.gov/drugs/
We are ready to serve !
 Uzbekistan
UzbekistanThe Pharmaceutical Industry Development Agency
- TBD after pilot
- DataMatrix
- https://uzpharmagency.uz/
We are ready to serve !