Solutions
Evidnt
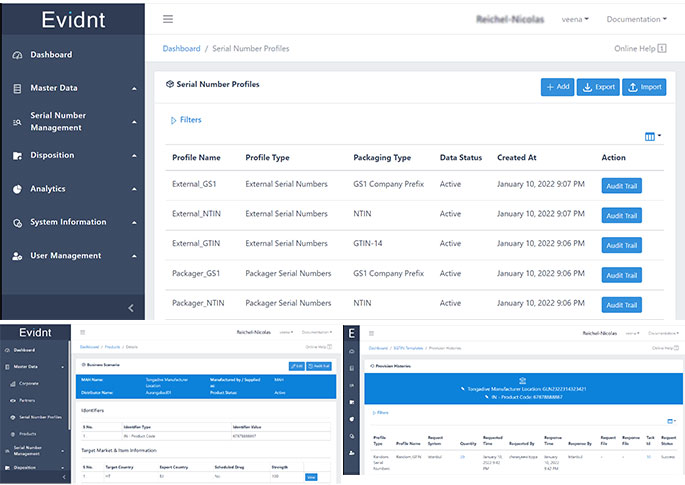
Evidnt by Tongadive is the most comprehensive end to end supply chain visibility software combining GS1 Serialisation, Blockchain DLT and Neural Networks to create a secure and immutable digital twin of the product package. This has far reaching applications across industries like Pharmaceutical, Medical Devices, Agriculture, Food Processing and Sustainable Lifestyle products from Cosmetics and Jewellery to Clothing and High Fashion.
Evidnt can be deployed as a cloud based SaaS (Software as a Service) or as On-Prem integrated system.
Where does Evidnt Add Value
Regulatory Compliance on supply chain visibility in regulated industries like Pharmaceutical, Medical Devices, Agriculture and Food Processing

L4, L5 and Next Generation supply chain visibility enabling regulatory compliance in industries like Pharmaceutical, Medical Devices, Agriculture and Food Processing

Cost efficiency through end to end supply chain visibility supporting ‘Just in Time’ production through better demand forecasting

Circularity and Re-commerce through trustworthy product origin information

Ethical and sustainable sourcing promotion through Provenance tracking
Unique Features of Evidnt

Unique Algorithm
Innovative algorithm that generates GS1 Serialised data and tracks it on Blockchain DLT Platform with an ability to run intelligent analytics and connection to Neural Networks based predictive demand forecasting information.

IT Systems
No fuss Deployment with plug & play approach supported by Tongadive expert team without having to make extensive changes in existing enterprise systems

User Experience
Simple and Intuitive end user experience making track and trace operations fun and less prone to errors

Automation
Compliance Audit on a Click enabled through automations reducing time and complexity of adherence to audit requirements and exposure to penalties

Flexibility/ Adaptability
Flexibility to adapt and tailor to supply chain stakeholder requirements through Cloud based microservices architecture
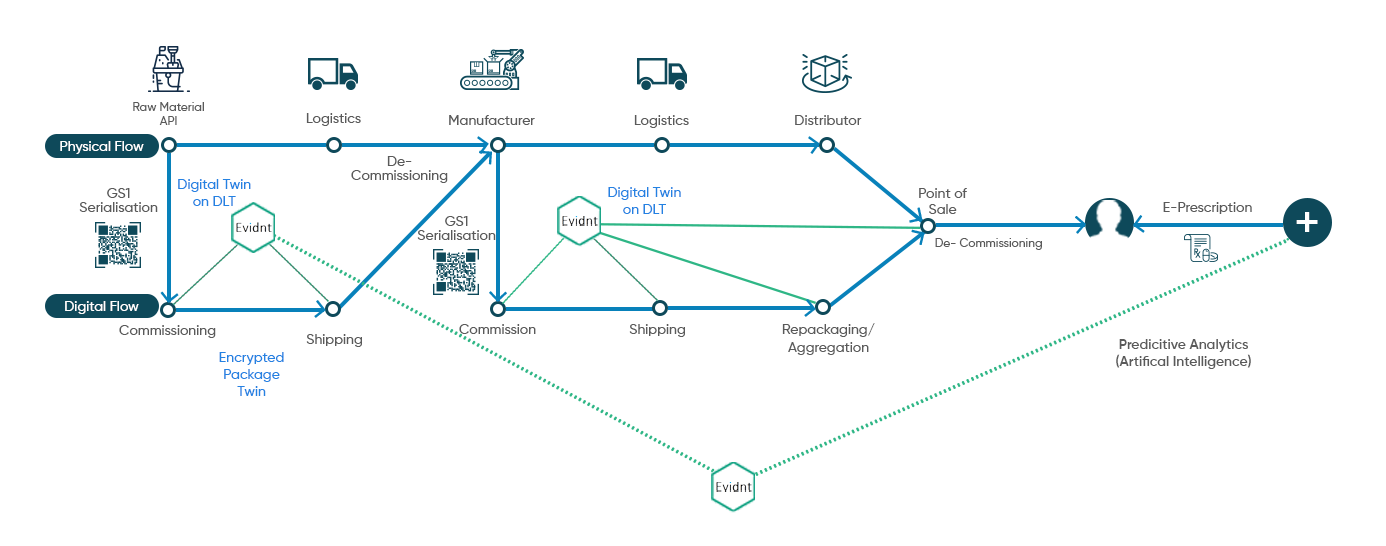
Digital Twin based Journey Tracking
Evidnt helps create a digital twin of product package and tracks
its journey from the source to the
end consumer and beyond.
Consulting and IT Services

GS1 Serialisation based Compliance
With extensive experience in digital technologies, compliance requirements across different sectors and countries and deep industry experience, Tongadive can provide a range of services to organisations to prepare for track and trace requirements that enables them to extend operations to new countries or adhere to new requirements in existing countries. Tongadive’s tailored services help develop and operationalise Serialisation strategies for a host of compliance requirements.
- US Drug Supply Chain Security Act (DSCSA)
- European Falsified Medicine Directive (FMD)
- UK Medicines and Healthcare Regulatory Agency (MHRA)
- India iVEDA portal (Integrated Validation of Exports of Drugs from India and its Authentication)
- Other Existing and Emerging Compliance Markets
Track and Trace based Packaging Solutions
Tongadive experts can help develop and operationalise track and trace strategy aimed at achieving business varied objectives.
- Visibility of products across supply chain
- Counterfeit avoidance strategies
- Cost efficiency in supply chain operations
- Demand forecasting for optimising production
- Supply chain software deployment support
- L1-L5 packaging and track and trace strategies
- Track and trace managed services
- Sustainability and re-commerce focused track and trace strategies
- Circularity protocols
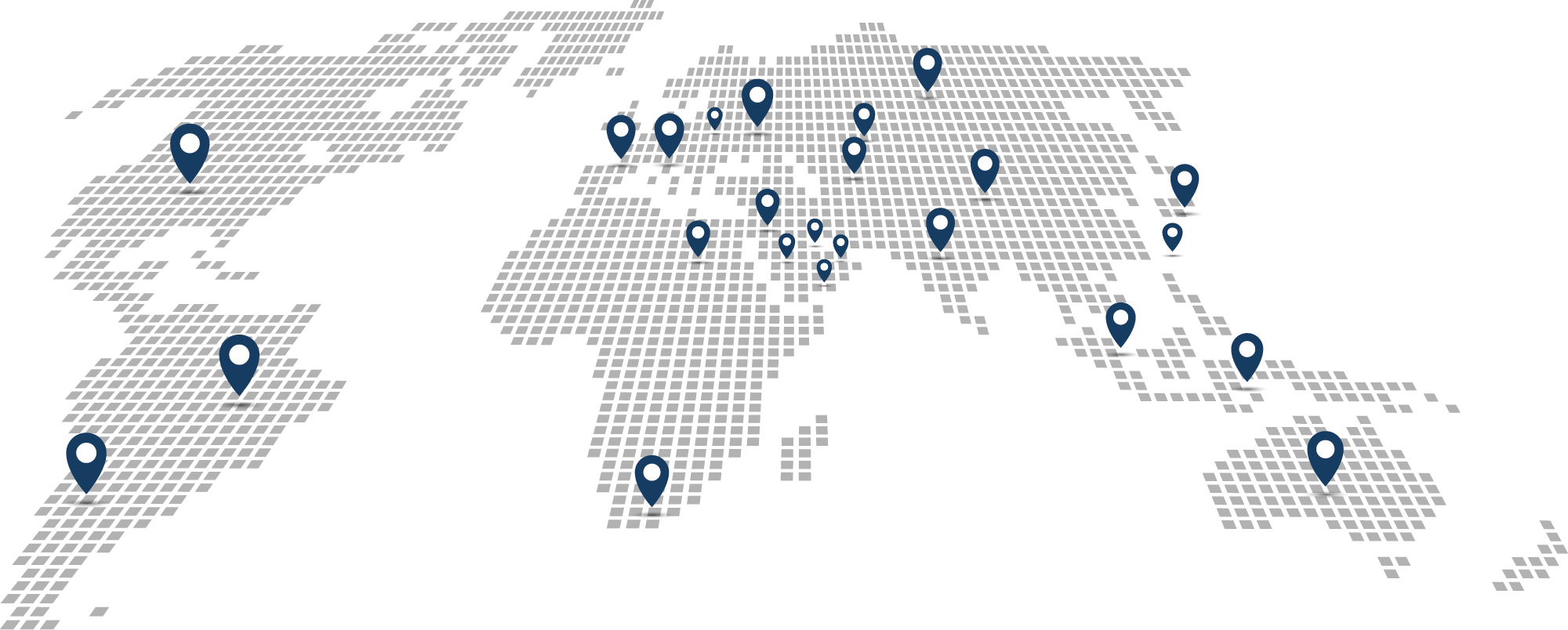
Serialization. Traceability. Compliance
 Argentina
ArgentinaNational Administration of Drugs, Foods & Medical Devices of Argentina (ANMAT)
- Required
- GS1-128, DataMatrix, RFID
- https://www.argentina.gob.ar/anmat
We are ready to serve !
 Australia
AustraliaDepartment of Health, Therapeutic Goods Administration
- Must comply by Jan. 1, 2023
- DataMatrix
- https://www.tga.gov.au
We are ready to serve !
 Bahrain
BahrainNational Health Regulatory Authority (NHRA)
- Required
- DataMatrix
- https://www.nhra.bh/
We are ready to serve !
 Brazil
BrazilBrazilian Health Regulatory Agency (ANVISA)
- Must comply by April 1, 2022
- DataMatrix
- https://www.gov.br/
We are ready to serve !
 China
ChinaNational Medical Products Agency (NMPA)
- No deadline announced
- Linear barcode, DataMatrix, RFID, HRI
- http://english.nmpa.gov.cn/
Coming Soon !
 Egypt
EgyptCentral Administration for Pharmaceutical Affairs (CAPA)
- No deadline announced
- DataMatrix
- http://www.eda.mohp.gov.eg/
We are ready to serve !
 India
IndiaMinistry of Commerce & Industry
- Required now
- DataMatrix
- https://commerce.gov.in/
We are ready to serve !
 Indonesia
IndonesiaNational Agency of Drug and Food Control
- Must comply by Jan. 1, 2023
- QR Code or DataMatrix
- GTIN or AI (distribution permit number) Serial number Expiry date…
GTIN or AI (distribution permit number)
Serial number
Expiry date
Batch/LotRequirements at tertiary, secondary & primary (including SN) levels
Requirement on primary packaging only if there is not an anti-tampering device on the packaging level above
- https://www.pom.go.id/new/home/en
We are ready to serve !
 Japan
JapanMinistry of Health, Labor, and Welfare
- No deadline announced
- N/A
- https://www.mhlw.go.jp/english/
We are ready to serve !
 Kazakhstan
KazakhstanMinistry of Healthcare
- No deadline announced
- DataMatrix
- https://www.gov.kz/
We are ready to serve !
 Lebanon
LebanonMinistry of Health MedTrack
- Does not seem required
- DataMatrix
- https://www.moph.gov.lb/en/
We are ready to serve !
 Malaysia
MalaysiaMinistry of Health of Malaysia
- Must comply by Jan. 1, 2023
- DataMatrix
- https://www.moh.gov.my/
We are ready to serve !
 Qatar
QatarMinistry of Public Health
- Does not seem required
- DataMatrix (secondary); DataMatrix or GS1 128 (tertiary)
- https://www.moph.gov.qa/
We are ready to serve !
 Saudi Arabia
Saudi ArabiaSaudi Food & Drug Authority (SFDA)
- Active
- DataMatrix
- https://www.sfda.gov.sa/en
We are ready to serve !
 South Africa
South AfricaNational Department of Health
- Active
- DataMatrix
- https://www.health.gov.za/
We are ready to serve !
 Switzerland
SwitzerlandSwiss Medicines Verification Organization (SMVO)
- Active
- DataMatrix
- https://smvo.ch/en/
We are ready to serve !
 United Arab Emirates
United Arab EmiratesMinistry of Health and Prevention
- Active
- DataMatrix
- https://mohap.gov.ae/en/
We are ready to serve !
 Ukraine
UkraineMinistry of Eduction and Science
- No deadline announced
- DataMatrix
- https://mon.gov.ua/eng
We are ready to serve !
 United States
United StatesFood and Drug Administration
- Must comply by Nov. 27, 2023
- DataMatrix
- https://www.fda.gov/drugs/
We are ready to serve !
 Uzbekistan
UzbekistanThe Pharmaceutical Industry Development Agency
- TBD after pilot
- DataMatrix
- https://uzpharmagency.uz/
We are ready to serve !